How Bacteria Digest Oil
Pure water is an asset we take for granted. Yet the transparent seawater of a coral reef may have emerged from a factory, a feed lot or an urban sewer. Alaska's seawater would also be clear except for the tides whisking up sediment from her many glaciers.
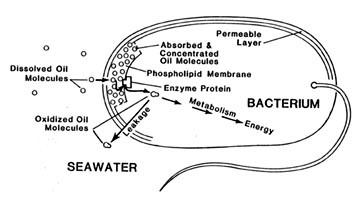
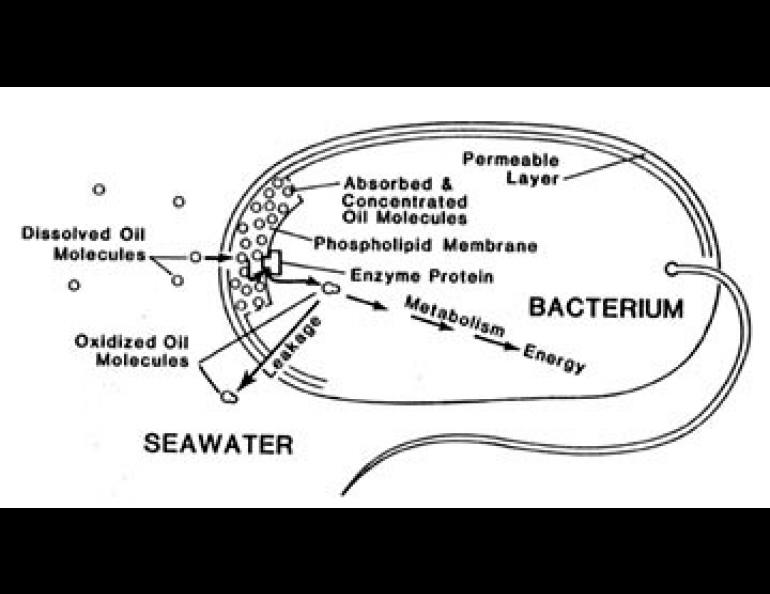
Microorganisms are responsible for this cleaning. The main problem they have in purifying water is to gather the tiny amounts of wastes dissolved in large amounts of water. This collection must be done fast enough for them to avoid starvation.
The process by which bacteria accumulate nutrients, wastes in this case, is called active transport. It is the same process that keeps our body fluids separated. Until recently, there were only two classes of active transport systems known. Now experiments at the Institute of Marine Science have led to the discovery of a third system which is especially suited for metabolizing oil.
These hydrocarbons are actively transported into bacteria for food and energy. Like other cells, the bacteria are surrounded by a layer of a grease-like substance, called phospholipid. Oil molecules prefer this layer to water and dissolve into it. Attractive forces between the water molecules squeeze the oil from the water into the membrane, thus driving the process. Enzymes on the inner surface of the membrane layer can then begin to oxidize and remove the oil. This product of oxidation is quite soluble in water so it can pass easily into the aqueous interior of the cell. Some of it remains there to provide food for the bacterium.
But a defect in the transport process, at least for aromatic hydrocarbons, causes most of the oil to be lost to the environment after first oxidation step. This is because these early products of oxidation are still oily enough to slip back out through the membrane. Re-gathering these lost products of hydrocarbon metabolism is done by one of the other better known transport systems, although these seem to be relatively inefficient.
The difference these oxidation products make to marine biology is not yet understood, but their existence is a good example of how major predictions about the environment can be made from studies on a molecular scale.