How Dry I Am
Paint is flaking off the walls. Your sinuses feel as if they were lined with a thin layer of concrete. House plants long for a vacation in the relatively moist Sahara. You stop slathering goop on cracking skin long enough to listen to the weather report, only to hear, "current temperature minus twenty degrees, relative humidity seventy-five percent." Obviously, the weather service's humidity has very little to do with the humidity where you live! But why?
Actually, if you measured the relative humidity outdoors, as the weather service does, you'd probably come up with about the same value they get. Contrary to general belief, Alaska north of the Coast Range complex does not have a particularly "dry" cold in winter. The only reason the humidity is not closer to 100 percent is because of the way relative humidity is defined. It's the amount of water actually in the air divided by the maximum amount the air could hold if it were saturated, that is, if it had an endless reservoir of liquid water to supply moisture. This is a perfectly good definition at temperatures above freezing. Ice, however, turns out to be less effective as a moisture source than supercooled water (water which stays liquid below freezing temperatures), and in Alaska we tend to have an endless reservoir of ice, rather than water, in the winter. Our normal winter humidities near the ground are generally close to the maximum possible in the presence of ice.
Why, then, are we and our houses so dehydrated through most of the winter as to threaten spontaneous combustion?
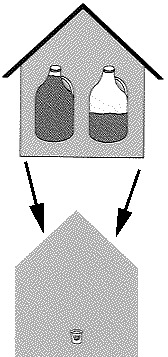
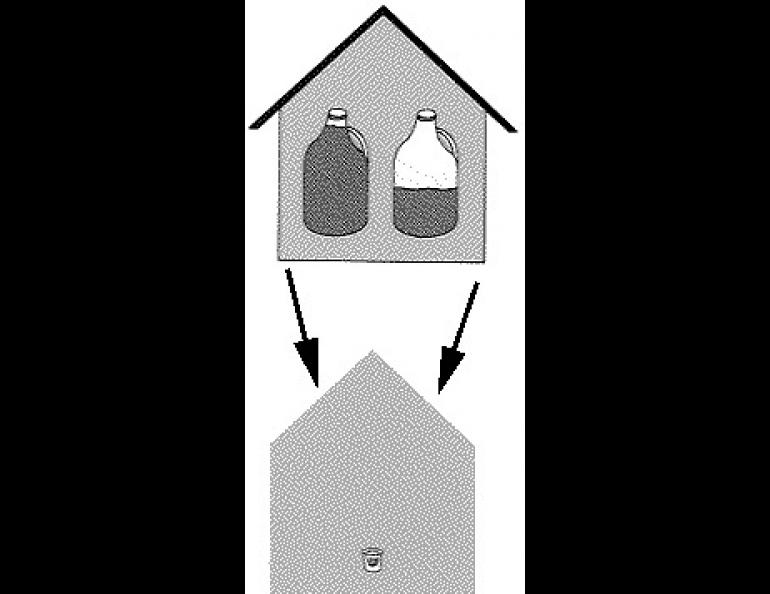
The amount of water that a given amount of air can hold depends on the temperature of the air as well as on whether the air is in contact with ice or water. The air in a moderate size house -- about 1325 square feet with eight-foot ceilings -- would hold almost a gallon and a half of water at 68 degrees F. At 32 degrees F, the same amount of air could hold only about a quart and a half of water. As temperatures drop, the amount of water gets less -- one and a quarter cups at zero, less than half a cup at twenty below, and a couple of tablespoonsful at forty below.
Now suppose we are maintaining the house temperature at 68° F. The air in the house is capable of holding almost a gallon and a half of water. But the same amount of air at outdoor temperatures is able to hold much less, especially if the effect of snow is considered. At forty below, for instance, an amount of outdoor air sufficient to fill the house, with an official relative humidity of 68 percent, will contain less than half a shot glass of water. When this outdoor air is brought into the house and warmed up to 68 degrees, it will still contain only half a shot glass of water, but will now be able to hold a gallon and a half, so the relative humidity of the outdoor air brought in and heated with no change in its water content will be about half of one percent. No wonder it is dry indoors in the winter!
Of course normal human activities -- cooking, bathing, even breathing -- are constantly adding water to the air indoors, and generally considerably more than is brought in with fresh air from outdoors. But beyond a certain point this extra water is removed by contact with cold windows and insulation. That, however, is a subject for a future column.