Don't Fill Her Up with Antifreeze
With winter approaching, most of us have begun to think about checking our antifreeze. If we're "good" down to, say, 20 below, it's probably wise to put in some more. How much? Well, on the back of most antifreeze cans or jugs, there is usually a table explaining the freezing points of different concentrations of antifreeze and water. Almost all of these tables show values down to about 60 below with a half-and-half mixture, but go no further. That's a pity, because it is at that point that strange things begin to happen.
It's not funny to the poor guy who wants to make really sure this winter, and with a "what the heck" attitude, fills the cooling system with pure antifreeze. He will be the one walking back into the house some frosty morning when its 10 below, shaking his head because his car radiator is frozen solid.
Thom Wigle of Dow Chemical in Ontario informs me that his office receives several hundred complaints each winter from irate customers complaining that their antifreeze is "no damned good." A typical story is that the customer was using a Dow product undiluted and their engine froze up at around zero.
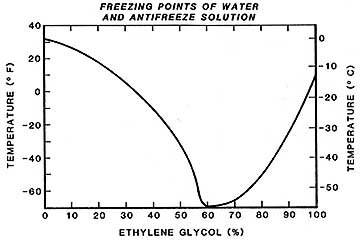
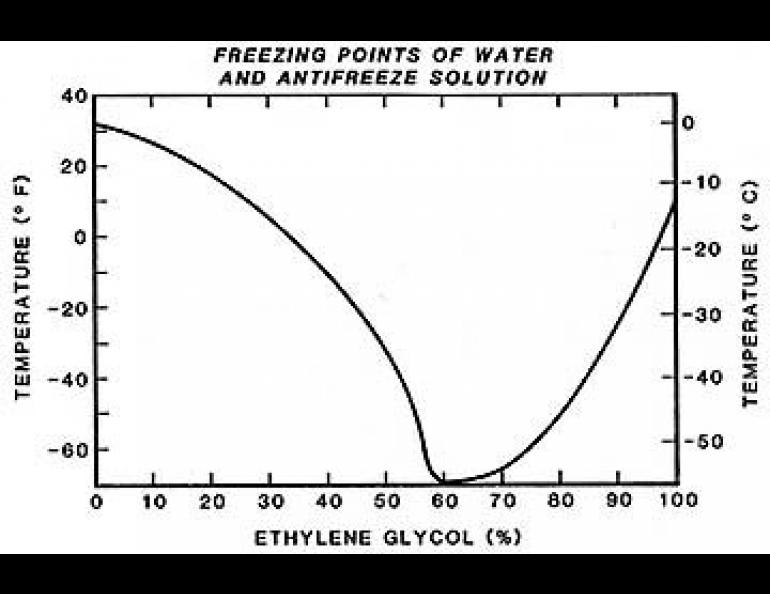
Actually, this is to be expected when one considers that ethylene glycol, the principal component of most antifreezes, freezes at 8 degrees above zero, Fahrenheit. It is only when water is added that the freezing point is depressed. The freezing point of an ethylene glycol and water mixture drops rapidly as the concentration of glycol is increased to a mixture of about 60% antifreeze and 40% water. Around that point, an abrupt turnabout occurs, and as more antifreeze is added, the freezing point rises almost as fast as it had previously dropped.
It's clearly a case of what you don't know can hurt you, but I have never seen an antifreeze container with an explanatory note to this effect.
Glycols do not have sharp freezing points, and even below the freezing temperatures, a slushy solution exists which will still flow. In the never-never transition zone around -60°F and 60% glycol, the mixture can either crystallize like water (particularly when "seeded" by a crystal and agitated) or set to a glass-like solid with no orderly internal crystalline structure. Either way, the result is the same, and thawing measures including strong language are prescribed.